- Aug 9
- 5 min
Electrocardiography - Part 3: Tachyarrhythmias
In Part 1 of this blog series on electrocardiography, we covered the basics of performing ECGs and looked at normal cardiac rhythms. In Part 2, we looked at the different types bradyarrhythmias. In this final post, we will explore tachyarrhythmias and review corresponding treatment considerations.
Sinus Tachycardia
A sinus tachycardia occurs due to increased automaticity of the SA node, and can be recognized electrocardiographically when all criteria for sinus rhythm are present however the heart rate is considered tachycardic (i.e., > 180 bpm in dogs or > 240 bpm in cats). A sinus tachycardia is not considered pathologic and is the result of another influence resulting in increased sympathetic tone. Common causes include excitement, stress, fever, pain, hyperthyroidism, anemia, hypovolemia, severe cardiac disease, and administration of positive chronotropic medications (i.e., terbutaline, theophylline, atropine, ketamine, dobutamine/dopamine). Treatment should be aimed at correction of the underlying cause, if necessary.
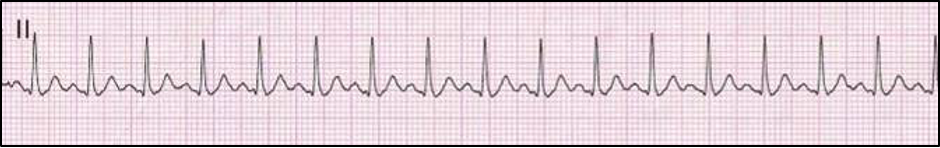
Atrial Premature Complexes (APCs) and Supraventricular Tachycardia (SVT)
Ectopic supraventricular arrythmias resulting in premature complexes or tachycardia are typically associated with atrial myocardial disease. This typically includes atrial stretch (due to AV valve disease or DCM), but also atrial tumors and pericardial disease. Occasionally, such arrhythmias are seen in patients with a primary cardiac electrical disturbance (i.e., accessory pathway), electrolyte imbalances, or non-cardiac contributing causes (i.e., splenic disease, pheochromocytoma). On surface ECG tracings, atrial premature complexes demonstrate an abnormal P-wave conformation (called a P’ wave) or an indiscernible P-wave due to blending with the preceding T-wave. Associated QRS complexes typically demonstrate normal morphology due to normal activation of the AV node, His-Purkinje system, and then ventricles, despite abnormal atrial activation. In most cases, this can be easily distinguished from ventricular ectopy, which results in wide and bizarre QRS complexes due to lack of use of the normal His-Purkinje system.
With the detection of greater than three atrial ectopic complexes in rapid succession, supraventricular tachycardia (SVT) is present. Supraventricular tachycardia can be difficult to distinguish from sinus tachycardia, however is it characterized by an abrupt onset and termination (compared to sinus tachycardia which is more gradual) and is typically much faster than expected from a sinus tachycardia (i.e., > 240 bpm in dogs; > 270 bpm in cats). Patients affected with intermittent APCs rarely demonstrate clinical signs, however SVT may contribute to decreased cardiac output with associated symptoms of lethargy and syncope. Chronic SVT can depress ventricular function resulting in a DCM-like status with possible CHF.
Treatment of infrequent, isolated APC’s is typically not necessary. In patients with associated supraventricular tachycardia, negative chronotropic therapy is advised. With emergent SVT resulting in symptoms of severely decreased cardiac output (i.e., collapse, HR > 300 bpm, severe hypotension), vagal maneuvers should be tried first (i.e., carotid body massage, eyeball pressure). If ineffective, intravenous diltiazem, beta-blocker therapy, or procainamide may be required. Given the lack of availability of oral procainamide, diltiazem is often considered the first-line choice. Intravenous beta-blockers (i.e., esmolol or propranolol) may be required to due to refractory tachycardia, however should be used with caution due to significant negative inotropic effects as many patients with SVT have severe myocardial disease. Similarly, oral therapy for SVT often consists of diltiazem, digoxin, sotalol, or atenolol.
%20and%20Supraventricular%20Tachycardia%20(SVT).png)
Did you know that Oncura Partners has a dedicated cardiology team that can review your ECGs? Click here to request more information about our electrocardiography support services.
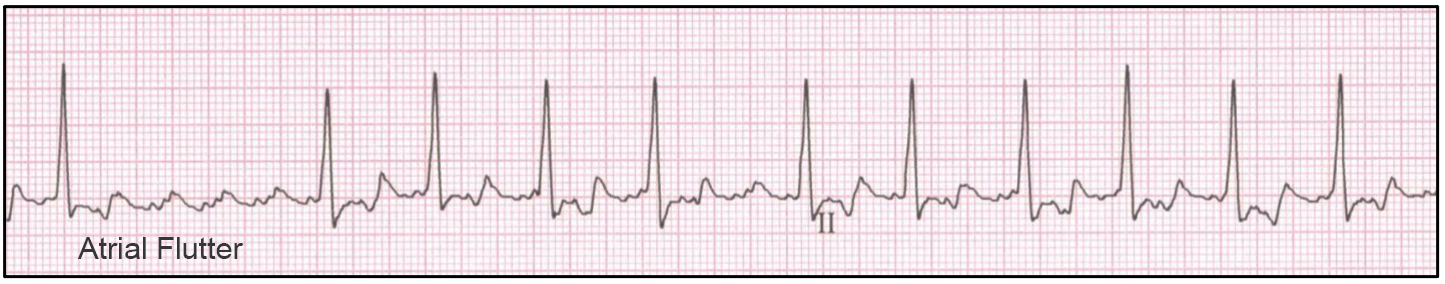
Atrial Fibrillation (AF) and Atrial Flutter
Atrial fibrillation and atrial flutter are unique forms of supraventricular tachycardia that result in chaotic depolarization of the atrial myocardium with associated lack of coordinated atrial contraction. Atrial fibrillation occurs due to multiple micro-reentrant circuits within the atria producing no discernible P-waves and a ‘quivering’ baseline (called ‘F’ waves). Other characteristics include an irregularly irregular pattern of QRS-T complexes with relatively normal conformation (due to the supraventricular origin) that are typically tachycardic in nature.
Atrial flutter shares many features of atrial fibrillation including an irregularly irregular rhythm, supraventricular originating QRS complexes, and tachycardia, however due to a macro-reentrant circuit atrial activity is seen as repeatable ‘f’ waves creating a characteristic ‘sawtooth’ baseline. The proportion of ‘f’waves followed by QRS complexes is a function of AV nodal refractoriness (i.e., 1:1, 2:1, 3:1 represents the number of ‘f’waves to QRS complexes).
Atrial fibrillation and flutter occur typically due to atrial stretch from structural cardiac disease (i.e., degenerative valve disease or DCM). In general, these rhythm disturbances are more likely with increases in atrial mass, and therefore large breed dogs are much more susceptible particularly with myocardial disease. Some giant breed dogs can develop atrial fibrillation with no significant structural heart disease which is termed ‘primary’ or ‘lone’ atrial fibrillation (similar to horses). Rarely, atrial fibrillation occurs in patients with normal cardiac chamber size due to increases in vagal tone (typically due to GI disease).
Treatment of atrial fibrillation and flutter is typically identical with two main treatment methods: rhythm control or rate control. Rhythm control is typically employed in patients without severe atrial enlargement and involves chemical or electrical cardioversion. As most patients display atrial enlargement that often results in return to atrial fibrillation following attempts at cardioversion, rate control is a more common goal when structural heart disease is present. This is also true for atrial flutter. Rate control therapy is aimed at reducing the ventricular rate associated with these arrhythmias by slowing conduction through the AV node. A realistic goal is a reduction in the ventricular rate to less than 150 bpm. This most commonly involves use of digoxin (0.002-0.003 mg/kg PO q 12 h) and diltiazem (1.0-1.5 mg/kg PO q 8 h or 2.5-3.5 mg/kg of extended release PO q 12 h) as combination therapy, however the potassium channel blocker sotalol (1.0 mg/kg PO q 12 h) or beta-blocker atenolol (0.25 mg/kg initially PO q 12 h) may be used as monotherapy at times. Similar to SVT, beta-blockers should be used with caution as these therapies are also potent negative inotropes and many patients with such atrial tachyarrhythmias have severe myocardial disease.
_Labeled.png)
Ventricular Premature Complexes (VPCs) and Ventricular Tachycardia (VT)
Ventricular premature complexes and ventricular tachycardia are relatively common arrhythmias that can be seen with not only structural cardiac disease but also extra-cardiac influences. The cardiac diseases most commonly associated include dilated cardiomyopathy (DCM) and Arrhythmogenic Right Ventricular Cardiomyopathy (ARVC), formerly referred to as ‘Boxer’ Cardiomyopathy. Although the exact mechanism remains unknown, extra-cardiac diseases including splenic disease, gastric dilatation volvulus (GDV), liver disease, pancreatitis, and sepsis, amongst others, can also result in ventricular ectopy.
The decision to initiate anti-arrhythmic therapy for ventricular arrhythmias is complex and not always straightforward. In general, ventricular tachycardia or frequent multiform VPCs in the presence of underlying cardiac disease should be treated. In patients with sustained ventricular tachycardia or associated hemodynamic consequence (i.e., hypotension, frequent collapse), emergent therapy may be necessary. In-hospital treatment typically involves use of bolus lidocaine therapy (in 1-2 mg/kg IV increments over 5-10 minutes to a total of 6-8 mg/kg) until cardioversion or adequate rate reduction is achieved. If necessary, a lidocaine CRI can be initiated thereafter (25-100 mcg/kg/min IV). When lidocaine is deemed ineffective, procainamide bolus therapy (6-12 mg/kg IV) can be attempted followed by a CRI (20-40 mcg/kg/min). As lidocaine and procainamide are both sodium channel blockers, toxicity of these drugs is additive and treatment should be separated by 30-60 minutes, if possible. Symptoms of sodium channel blocker toxicity include nausea, vomiting, ataxia, and seizures (diazepam responsive). Oral therapy for ventricular ectopy most commonly includes sotalol (1-3 mg/kg PO q 12 h) and/or mexiletine (4-8 mg/kg PO q 8 h with food). As sotalol has beta-blocking properties, it should be used with greater caution in patients with suspected dilated cardiomyopathy due to negative inotropic effects. Directed treatment at an extra-cardiac morbidity expected to contribute to VPCs and VT should always be performed, if possible.
%20and%20Ventricular%20Tachycardia%20(VT).png)
Accelerated Idioventricular Rhythm (AIVR)
An accelerated idiovenricular rhythm (AIVR) is a ventricular rhythm that is sometimes referred to as ‘slow V-tach’. This rhythm occurs when there are foci of ventricular ectopy that exceed the sinus rate but are not tachycardic in nature (typically 100-160 bpm in the dog). Most commonly, AIVR occurs due to extra-cardiac disease such as splenic tumors, GDV, pancreatitis, sepsis, etc., however can also be present with structural cardiac disease. Due to the lack of tachycardia, this rhythm often does not result in hemodynamic consequence. Treatment should be aimed at correction of the suspected underlying cause, however if progression to VT occurs, therapy is advised as above.
.png)
